A Simple, Quantitative, Reproducible Method
Gino Soldati, MD, Andrea Smargiassi, MD, PhD , Riccardo Inchingolo, MD , Danilo Buonsenso, MD ,
Tiziano Perrone, MD, PhD, Domenica Federica Briganti, MD, Stefano Perlini, MD, PhD, Elena Torri, MD,
Alberto Mariani, MD, Elisa Eleonora Mossolani, MD, Francesco Tursi, MD, Federico Mento, MSc ,
Libertario Demi, PhD
Adecade of clinical and physical studies clearly showed thatlung ultrasound (LUS) is able to detect interstitial lung disease,subpleural consolidations, and acute respiratory distresssyndrome from any etiologic cause.
New evidence from publishedstudies, national and international organization statements, andinformal case discussions with internationally recognized experts areshowing the usefulness of LUS for the management of patients with2019 new coronavirus disease (COVID-19) pneumonia, fromdiagnosis to monitoring and follow-up.
To date, available medical treatments for COVID-19 pneumoniainclude anti–human immunodeficiency virus drugs, idrossicloroquine,ventilatory support, prone positioning, and extracorporeal membranetherapy for critical patients.
However, recent findings suggest thatanti–interleukin 6 monoclonal antibodies can be useful in blockingthe inflammatory cascade involved in lung inflammation duringCOVID-19 infection. Evidence also suggests that the earlier we treat,the better patients improve with treatment.
Therefore, LUS couldbe useful, being performed at several time points from clinicaldiagnosis, in determining early lung involvement during thepaucisymptomatic phase of the disease and potentially playing a rolein treatment decisions.
Funding organizations are starting to supportclinical trials; in this regard, LUS can be used to monitor lunginvolvement during a specific treatment. Importantly, LUS can beused in every setting, including low- to middle-income countries, allowingthe reduction of disparities in trial participation, since secondary-level imaging studies (such as computedtomography) are not easily accessible everywhere.
Therefore, this global emergency needs a globalunified approach, with all researchers speaking the samelanguage. For this reason, we propose a standardizationfor the international use of LUS for the management ofpatients with COVID-19.
Our LUS COVID team is made up of Italianexperts in LUS currently involved in the clinical managementof COVID-19 in different Italian areas,including the heavily involved cities of northern Italy.
Moreover, experts in ultrasound (US) physics andimage analysis are part of the team.The team developed a standardized approachregarding equipment and the acquisition protocol.Moreover, the team proposed a scoring system forseverity classification.
To this aim, clinicians shared30 cases of confirmed COVID-19 in an anonymizedvirtual database, for a total of about 60,000 frames todate. All team members discussed their clinical casesthrough online meetings. Images were reviewed by allteam members, who were blinded to the clinical background,and listed in classes of the severity of lunginvolvement based on LUS images
At the end of thisprocess, a biomedical engineer expert in LUS collectedthe data and suggested a LUS grading systemfor COVID-19 pneumonia. Again, the biomedicalengineer resubmitted the images grouped in differentclasses of severity to the study members, who wereblinded of clinical data, to review the images againand evaluated agreement regarding the LUS scores.
The score was defined only when all team membersagreed.
Methods
In the setting of COVID-19, wireless transducers andtablets represent the most appropriate US equipment.
These devices can easily be wrapped in single-use plasticcovers, reducing the risk of contamination and makingsterilization procedures easy.
Such devices aremuch less expensive than usual US machines, includingthe portable ones.In cases of unavailability of these devices, portablemachines dedicated to exclusive use for patients with COVID-19 can be used, although maximum care forsterilization is necessary.
In these cases, transducer andkeyboard covers are suggested, and sterilization proceduresare necessary, following recent recommendations.
Sharing our real-world experience in performing LUS examinations in patients with COVID-19, wepropose 2 different ways of performing LUS examinationswith pocket devices, aiming to reduce the exposureof health workers to cases.
One operator uses the transducer performing theUS examination; the other one keeps the tablet andfreezes images/videos.
The second operator can beeither in the room, being at a safe distance from thepatient (about 2 m), or even remain outside the door,communicating by a phone call with the operator tooptimize the quality of images. Potentially, this lastapproach can reduce the operator dependence of theUS, since the second operator blindly selectsthe images, being unaware of the clinical condition ofthe patient.
The 2 operators will follow an agreed,tested, and standardized images acquisition protocol.
Acquisition Protocol
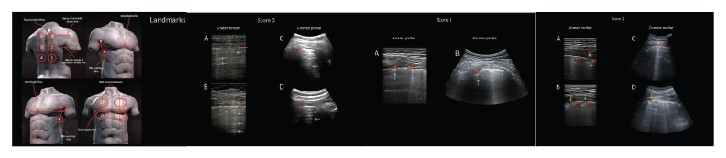
Fourteen areas (3 posterior, 2 lateral, and 2 anterior)should be scanned per patient for 10 seconds alongthe lines indicated here. Scans need to be intercostalto cover the widest surface possible with a single scan.A standard sequence of evaluations is proposed,using landmarks on chest anatomic lines (Figure 1).
Echographic scans can be identified with progressivenumbering starting from the right posterior basalregions. For a patient able to maintain the sittingposition:
- Right basal on the paravertebral line above thecurtain sign;
- Right middle on the paravertebral line at the inferiorangle of the shoulder blade;
- Right upper on the paravertebral line at the spineof the shoulder blade;
- Left basal on the paravertebral line above the curtainsign;
- Left middle on the paravertebral line at the inferiorangle of the shoulder blade;
- Left upper on the paravertebral line at the spineof the shoulder blade;
- Right basal on the midaxillary line below theinternipple line;
- Right upper on the midaxillary line above theinternipple line;
- Left basal on the midaxillary line below the internippleline;
- Left upper on the midaxillary line above theinternipple line;
- Right basal on the midclavicular line below theinternipple line;
- Right upper on the midclavicular line above theinternipple line;
- Left basal on the midclavicular line below theinternipple line; and
- Left upper on the midclavicular line above theinternipple line.
In cases of performance of LUS examinations incritical care settings (such as patients receiving invasiveventilation) and for patients who are not able tomaintain the sitting position, the posterior areasmight be difficult to evaluate. In these cases, the operatorshould try to have a partial view of the posteriorbasal areas, currently considered “hot areas” forCOVID-19 and, however, start the echographicassessment from landmark number 7.
- Use convex or linear transducers, according to thepatient’s body size.
- Use a single–focal point modality (no multifocusing),setting the focal point on the pleuralline. Using a single focal point and setting it at theright location have the benefit of optimizing thebeam shape for sensing the lung surface. At thefocus, the beam has the smallest width and is thereforeset to best respond to the smallest details.
- Keep the mechanical index low (start from 0.7 andreduce it further if allowed by the visual findings).High mechanical indices, used for a long observationtime, may result in damage to the lung.
- Avoid saturation phenomena as much as possible;control gain; and diminish the mechanical index ifneeded (see examples of LUS images in the figures).

Saturation phenomena occur, eg, when thesignal strength of the echo signals is too high forthe receiving electronics to be converted into electricalsignals, conserving a linear relationship withthe pressure amplitude. This has the effect of distortingthe signals and produces images where thedynamics of the actual signal are lost. The visualappearance of this phenomenon is the presence of areas that are completely white. In this case, it istherefore not possible to appreciate local variationsin the response to insonations.
- Avoid the use of cosmetic filters and specific imagingmodalities such as harmonic imaging, contrast,Doppler, and compounding.
- Achieve the highest frame rate possible (eg, no persistenceand no multifocusing).
- Save the data in the Digital Imaging and Communicationsin Medicine format. In case this is notpossible, save the data directly as a video format.Visual findings, especially when related to verysmall alterations, do not appear on every frame. Itis thus advantageous to acquire videos, where thelung surface below the landmark can be monitoredfor a few seconds during breathing.
Scoring Procedures
- Score 0: The pleural line is continuous and regular.Horizontal artifacts are present. These artifacts aregenerally referred to as A-lines (See Figures 2–5).They are due to the high reflectivity of the normallyaerated lung surface and characterize thevisual representation of the multiple reflectionshappening between the US transducer and the lungsurface itself.3,5,7
- Score 1: The pleural line is indented. Below theindent, vertical areas of white are visible. These aredue to local alterations in the acoustical propertiesof the lung, as, for example, the replacement of volumespreviously occupied by air in favor of mediathat are acoustically much more similar to theintercostal tissue (water, blood, and tissue). Thisphenomenon opens channels accessible to US,which can explain the appearance of the verticalartifacts.3,5,7
- Score 2: The pleural line is broken. Below thebreaking point, small-to-large consolidated areas(darker areas) appear with associated areas of whitebelow the consolidated area (white lung). Thedarkening of the consolidated areas signals the lossof aeration and the transition of these areas towardacoustic properties similar to soft tissue over theentire area represented by the consolidation itself.Beyond the consolidations, the appearance of areasof white lung signals the presence of areas not yetfully deaerated, where air inclusions are still presentbut embedded in tissuelike material. This highlyscattering environment can explain this peculiarpattern.3,5,7
- Score 3: The scanned area shows dense and largelyextended white lung with or without largerconsolidations.



At the end of the procedure, the clinician willwrite for each area the highest score obtained (eg,quadrant 1, score 2; quadrant 10, score 1; and so on).
International Database for Data Storage, Image
Analyses, and Artificial Intelligence StudiesWe strongly encourage the scientific community toembrace the development of a protected, internationallyavailable database that allows uploading imagesand videos of patients with COVID-19 (radiography,US, and computed tomography).
This will speed thedevelopment of dedicated pattern recognition algorithmsable to recognize COVID-19–related pathologicfindings, allow for comparisons between differentcenters, and foster the development of telemedicineprograms (including remote evaluation of images,clinical advice, and case discussions) and telematicteaching programs.
Here is the link to our database: https://covid19.disi.unitn.it/iclusdb.
Discussion and Conclusions
COVID-19 is a worldwide health challenge, involvingnot only health but also economics and social behaviors
For the first time in the era of modern medicine,the whole world is facing the same threat. This cangive us the opportunity to change our researchapproach: the time has probably come to share ourknowledge and plan the best care altogether. The aimof this article is thus to share our experience and topropose a standardization with respect to the use of LUS in the treatment of patients with COVID-19.

The article introduces the detailing of landmarks and imaging settings to the acquisition protocol and presentsa scoring mechanism developed within ourgroup. We further emphasize the need for a shareddatabase, which is necessary to foster further developmentsand to disseminate the results achieved.
References
- Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasoundfor critically ill patients. Am J Respir Crit Care Med 2019; 199:701–714.
- Mayo PH, Copetti R, Feller-Kopman D, et al. Thoracic ultrasonography:a narrative review. Intensive Care Med 2019; 45:1200–1211.
- Soldati G, Demi M, Smargiassi A, Inchingolo R, Demi L. The roleof ultrasound lung artifacts in the diagnosis of respiratory diseases.Expert Rev Respir Med 2019; 13:163–172.
- Soldati G, Smargiassi A, Mariani AA, Inchingolo R. Novel aspectsin diagnostic approach to respiratory patients: is it the time for anew semiotics? Multidiscip Respir Med 2017; 12:15
- Demi M, Prediletto R, Soldati G, Demi L. Physical mechanismsproviding clinical information from ultrasound lung images:hypotheses and early confirmations. IEEE Trans Ultrason FerroelectrFreq Control 2020; 67:612–623.
- Demi L, van Hoeve W, van Sloun RJG, Soldati G, Demi M. Determination of a potential quantitative measure of the state of the lungusing lung ultrasound spectroscopy. Sci Rep 2017; 7:12746.
- Soldati G, Demi M, Inchingolo R, Smargiassi A, Demi L. On thephysical basis of pulmonary sonographic interstitial syndrome.J Ultrasound Med. 2016; 35:2075–2086. https://doi.org/10.7863/ultra.15.08023.
- Soldati G, Smargiassi A, Inchingolo R, et al. Lung ultrasonographyand vertical artifacts: the shape of air. Respiration 2015; 90:86.
- Soldati G, Smargiassi A, Inchingolo R, et al. Lung ultrasonographymay provide an indirect estimation of lung porosity and airspacegeometry. Respiration 2014; 88:458–468.
- Peng QY, Wang XT, Zhang LN; Chinese Critical Care UltrasoundStudy Group (CCUSG). Findings of lung ultrasonography of novelcorona virus pneumonia during the 2019–2020 epidemi [publishedonline ahead of print March 12, 2020]. Intensive Care Med. doi:https://doi.org/10.1007/s00134-020-05996-6.
- Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help criticalcare clinicians in the early diagnosis of novel coronavirus(COVID-19) pneumonia? Radiology 2020; 13:200847.
- Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lungultrasound during the COVID-19 pandemic [published onlineahead of print March 20, 2020]? J Ultrasound Med. doi:10.1002/jum.15284.
- Chen J, Qi T, Liu L, et al. Clinical progression of patients withCOVID-19 in Shanghai China [published online ahead of printMarch 19, 2020]. J Infect. https://doi.org/10.1016/j.jinf.2020.03.004.
- Buonsenso D, Pata D, Chiaretti A. COVID-19 outbreak: lessstethoscope more ultrasound [published online ahead of printMarch 20, 2020]. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30120-X.
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruseson inanimate surfaces and their inactivation with biocidalagents. J Hosp Infect 2020; 104:246–251.
- Miller DL, Dong Z, Dou C, Raghavendran K. Pulmonary capillary hemorrhage induced by different imaging modes of diagnosticultrasound. Ultrasound Med Biol 2018; 44:1012–1021.
PDF file
scarica PDF